J Res Health Sci. 24(4):e00626.
doi: 10.34172/jrhs.2024.161
Review Article
COVID-19 Vaccine Effectiveness of Booster Doses Against Delta and Omicron Variants Over Follow-up Times Using Longitudinal Meta-analysis
Farideh Mostafavi 1, 2  , Mansour Bahardoust 1, 2, Francesco Sera 3, Alireza Amirabadizadeh 4, Sepehr Allahyari 5, Paddy Ssentongod 6, Manochehr Karami 2, Seyed Saeed Hashemi Nazari 2, *
, Mansour Bahardoust 1, 2, Francesco Sera 3, Alireza Amirabadizadeh 4, Sepehr Allahyari 5, Paddy Ssentongod 6, Manochehr Karami 2, Seyed Saeed Hashemi Nazari 2, * 
Author information:
1Student Research Committee, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Department of Epidemiology, School of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Department of Statistics, Computer Science and Applications ‘G.Parenti’, University of Florence, Florence, Italy
4Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, 9717113163, Iran
5Department of Virology, Doctor of Veterinary Medicine Student, Faculty of Veterinary Medicine, Garmsar Branch, Islamic Azad University, Tehran, Iran
6Department of Medicine, Penn State Milton S. Hershey Medical Center, Hershey, PA 17033, USA
Abstract
Background: COVID-19 is a viral disease caused by the SARS-CoV-2, leading to several variants. This study aimed to examine the effectiveness of booster doses against the Delta and Omicron variants over different follow-up times.
Study Design: This was a longitudinal meta-analysis.
Methods: Searches were performed in PubMed, Cochrane Library, Scopus, and Web of Science databases, and eighty studies were selected for investigation. The analyses were separately performed on the unvaccinated control group (UNVCG) and the complete two doses of the vaccine control group (C2DCG) against Delta and Omicron variants. Three outcomes were examined, including symptomatic infection, hospitalization, and death.
Results: Vaccine effectiveness (VE) in UNVCG studies for symptomatic infection revealed a non-linear trend against Omicron with a peak of 67.3%, declining to 27.1% after 25 weeks after a booster dose. The mean of VE for hospitalization over time started to decrease after four weeks against Omicron and after eight weeks against Delta. The VE reached a peak at week eight (96.0%) and started to decline with a VE of 93.3% after 20 weeks after the booster dose against Delta. It was 90.8% at week four and decreased to 73.4% after 25 weeks after the booster dose against Omicron. VE in the C2DCG studies demonstrated more decreases in outcomes over time.
Conclusion: Our findings showed a tendency to decrease effectiveness over time based on outcomes and variants. The early protection levels were lower in Omicron. Moreover, the VE decrease over time was stronger in Omicron compared to the Delta variant.
Keywords: COVID-19 vaccines, Vaccine efficacy, COVID-19 vaccine booster shot
Copyright and License Information
© 2024 The Author(s); Published by Hamadan University of Medical Sciences.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Mostafavi F, Bahardoust M, Sera F, Amirabadizadeh A, Allahyari S, Ssentongod P, et al. COVID-19 vaccine effectiveness of booster doses against delta and omicron variants over follow-up times using longitudinal meta-analysis. J Res Health Sci. 2024; 24(4):e00626. doi:10.34172/jrhs.2024.161
Background
The main strategy of the health systems for controlling the acute stage of the COVID-19 epidemic was universal vaccination.1 Since the beginning of the epidemic, global efforts have focused on the development of safe and effective vaccines to prevent COVID-19. Before the epidemic, the development of a vaccine was a long and complex process that lasted several decades before the approval of its clinical use. Shortly after the onset of COVID-19, scientists began a race to develop an effective and safe vaccine against this disease based on new and old vaccine technologies. In less than two years, more than 300 vaccines worldwide were candidates, and 117 vaccines were developed in different clinical stages, of which 30 vaccines are in phase three studies. As of mid-2021, seven COVID-19 vaccines have been licensed for emergency use.1,2
Effectiveness data show high levels of short-term protection of COVID-19 vaccines against clinical diseases and severe outcomes, including hospitalization and death.3-5 However, there is evidence that protection against the disease decreases with time and against different virus variants.6 In late 2020 and 2021, a wide range of SARS-CoV-2 variants emerged, replacing the original Wuhan strain, some of which were associated with increased transmission and successive waves of infection in many countries.
Due to the decrease in the effectiveness of vaccines after the second dose and the occurrence of different variants of the virus, booster dose vaccination started in several countries. Studies are being implemented to evaluate the effectiveness of the third dose or booster in these countries.7
This study aims to integrate the published data regarding the effectiveness of the third dose or first booster vaccines to prevent symptomatic, severe disease, and death with a multivariate meta-analysis method using the main outcome set for the effectiveness of COVID-19 vaccines in clinical trials and observational studies that have been conducted so far. Our results may provide additional evidence-based information to help select the best policy to achieve increased coverage of booster dose vaccination against COVID-19 and reduce severe disease and mortality.
Methods
Search strategy
PubMed, the Cochrane Library, EMBASE, Scopus, and the Web of Science databases were searched from January 1, 2020, to March 25, 2023, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Three authors independently researched the databases, reviewed the relevant titles and summaries, and extracted the information required for data analysis. The authors and other experts were contacted for inadequate data.
The general search strategy included (((COVID 19[Title/Abstract] ) OR (SARS CoV 2 Infection[Title/Abstract])) OR (Coronavirus Disease 2019[Title/Abstract])) OR (COVID-19 Pandemic[Title/Abstract]) AND ((COVID-19 Vaccines[Title/Abstract]) OR (SARS-CoV-2 Vaccines[Title/Abstract])) OR (Coronavirus Disease-19 Vaccine[Title/Abstract]) AND ((Vaccine Efficacy[Title/Abstract]) OR (Vaccine effectiveness[Title/Abstract])) AND ((booster dose[Title/Abstract]) OR (booster shot[Title/Abstract])) OR (vaccine booster[Title/Abstract]).
All the retrieved studies were stored in databases using EndNote. Duplicate studies were removed using the automatic removal function of EndNote. In addition, for more certainty, some remaining repeated studies were removed manually. Unpublished studies, letters, or short communication were not included in the investigation.
Study selection
After screening the titles, the articles’ abstracts and full texts were reviewed until the studies that met the inclusion criteria were included in the study. Studies were included only if the booster dose and the two primary doses were specified in the articles, and the effectiveness of the booster dose vaccine was calculated. Clinical trials or observational studies that included the inclusion criteria and had sufficient data on the effectiveness of the third vaccine dose at defined time points against the Delta or Omicron variant were included in the study. On the other hand, studies that only examined the effectiveness of the first and second doses were excluded from the investigation.
Data extraction
Two researchers independently extracted and tabulated the key data from the studies. A third investigator was consulted if there was disagreement. The articles were initially screened by two independent researchers, and any differences in the selection of articles in the screening process were resolved in consultation with the third researcher. The extracted variables were the name of the first author, type of study, year of publication, sample size, the type of previous second dose and first booster dose, country, age, time since the injection of the booster vaccine dose (in the week), previous COVID-19 infection, dominant variant, and vaccine efficacy or vaccine effectiveness (VE).
Quality assessment
The quality or risk assessment of the cohort and case-control studies was performed by the Newcastle-Ottawa scale.8 This checklist evaluates the quality of studies in the selection, comparability, and outcome/exposure sections and assigns a star(s) for each item.8
The methodological quality and risk of bias of randomized controlled trial (RCT) studies were evaluated using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2).9 This involved assessing random sequence generation, allocation concealment, blinding of participants and healthcare personnel, blinded outcome assessment, completeness of outcome data, selective reporting, and other biases. Based on this tool, the studies were categorized into three levels of quality (low, some concerns, and high).
Statistical analysis
The effectiveness of the booster dose (third dose) was investigated for seven types of vaccines, including BNT162b2, Moderna, ChAdOx1, CoronaVac, Jansen, mRNA, and Sinopharm. The comparison group to calculate the VE was different in studies; in some studies, booster vaccination was compared with the complete two doses of the vaccine control group (C2DCG), and in other studies, booster vaccination was compared with the unvaccinated control group (UNVCG). Accordingly, analyses were performed separately in terms of the two control groups for Delta and Omicron variants. Three outcomes were investigated, including VE for symptomatic COVID-19 infection, COVID-19-related hospitalization, and COVID-19-related death.
In this review, some studies reported that the VE for deaths was 100% while not reporting the confidence interval (CI) and standard error. Firth’s regression was used to calculate the variance of these studies to include them in the analysis since there were zero deaths in the vaccine group, and the sample size was extremely small in these studies.
The results of each study were pooled at each time point to provide overall VE. Subgroup analyses were performed to identify heterogeneity sources across studies. In addition, the univariate meta-analysis random-effects model was implemented, allowing studies to have their own population effect sizes, according to the type of booster (BNT162b2, Moderna, ChAdOx1, CoronaVac, Jansen, mRNA, and Sinopharm), age (≥ 12 years old and ≥ 50 years old), study design (RCT, case control, negative case control, and cohort), previous COVID-19 infection (yes/no), and risk of bias (low, moderate, or high risk).
The univariate model assumes that the effect sizes are independent at different time points, so they ignore the correlation between effect sizes, and this can increase the standard error of the point estimates. To evaluate the VE over the follow-up period (1, 4, 8, 12, 16, 20, 24, and > 25 weeks), a longitudinal meta-analysis was performed, which assumes that the effect sizes at one time are correlated with the effect sizes at other times. The longitudinal structure of the meta-analysis at different time points, using a multivariate approach, can be defined as follows:
yi= Xiβ1+ bi + εi
which is a general linear mixed model. β1 is the vector of fixed effects at each follow-up time, and bi denotes the vector of random effects at each follow-up time. Further, εi represents the vector of residuals. In the multivariate model, random effects have a multivariate normal distribution with a zero mean and a covariance matrix D.10,11 In this analysis, the structure of the covariance matrix was considered autoregressive (AR), which is an extension of the independent random time effects model, where the dependence between effect sizes is measured by the dependence between random time effects. This model considers a heterogeneous AR(1) covariance structure for random time effects while assuming within-study serial correlations between longitudinal effect sizes, Si = diag (
). Consequently, the variance-covariance matrix is V(Yi) = ∑ + Si, diagonal elements (
), and off-diagonal elements (
). For time points t and
, ρτ is the correlation between any two adjacent random time effects.10 Therefore, the dependence between effect sizes increases with a decrease in the distance between them. This is appropriate in longitudinal studies where the loss of follow-up increases over time, so that the effect values measured more distant are less dependent than those measured closer together.
Publication bias was evaluated graphically with funnel plots and by the trim-and-fill, Egger’s test, and Bagg’s test. Trim-and-fill is a non-parametric approach that assesses the asymmetry of the funnel plot. The horizontal axis of the funnel plot indicates the effect sizes of studies, and the vertical axis denotes their measures of precision (e.g., standard errors, sample size, and the like). First, this method figures out the publication bias by detecting any asymmetry in funnel plots, then adjusts the bias by trimming or removing small studies, causing asymmetry in the plot to estimate the true center of the plot, and finally fills or replaces the removed studies and their missing ‘counterparts’ around the center.12
Egger’s test is based on a linear regression of the studies’ effect size on their standard errors or any other precision measures to quantify funnel plot asymmetry, in which a significant intercept indicates possible publication bias. Bagg’s test uses Kendall’s tau to evaluate whether there is a significant correlation between the ranks of studies’ effect size and the rank of their variance. The significance of Bagg’s test shows no selection bias present, and non-significant studies are less likely to be published 13. Meta-analysis was performed with Stata 17 and R software with the metafor package in R (version R-4.2.3).11
Results
Overall, 4163 studies from databases were identified for analysis. After removing duplicates, 1,730 studies were screened for eligibility in terms of abstracts and titles. After removing 1596 studies, 134 studies were checked in terms of full texts, and finally, 80 studies were included in the analysis (Figure 1). Out of the 80 studies, there were two RCT studies,14,15 eight case-control studies,16-23 33 negative case-control studies,24-56 and 37 cohort studies57-93 from 28 countries (Figure 1).
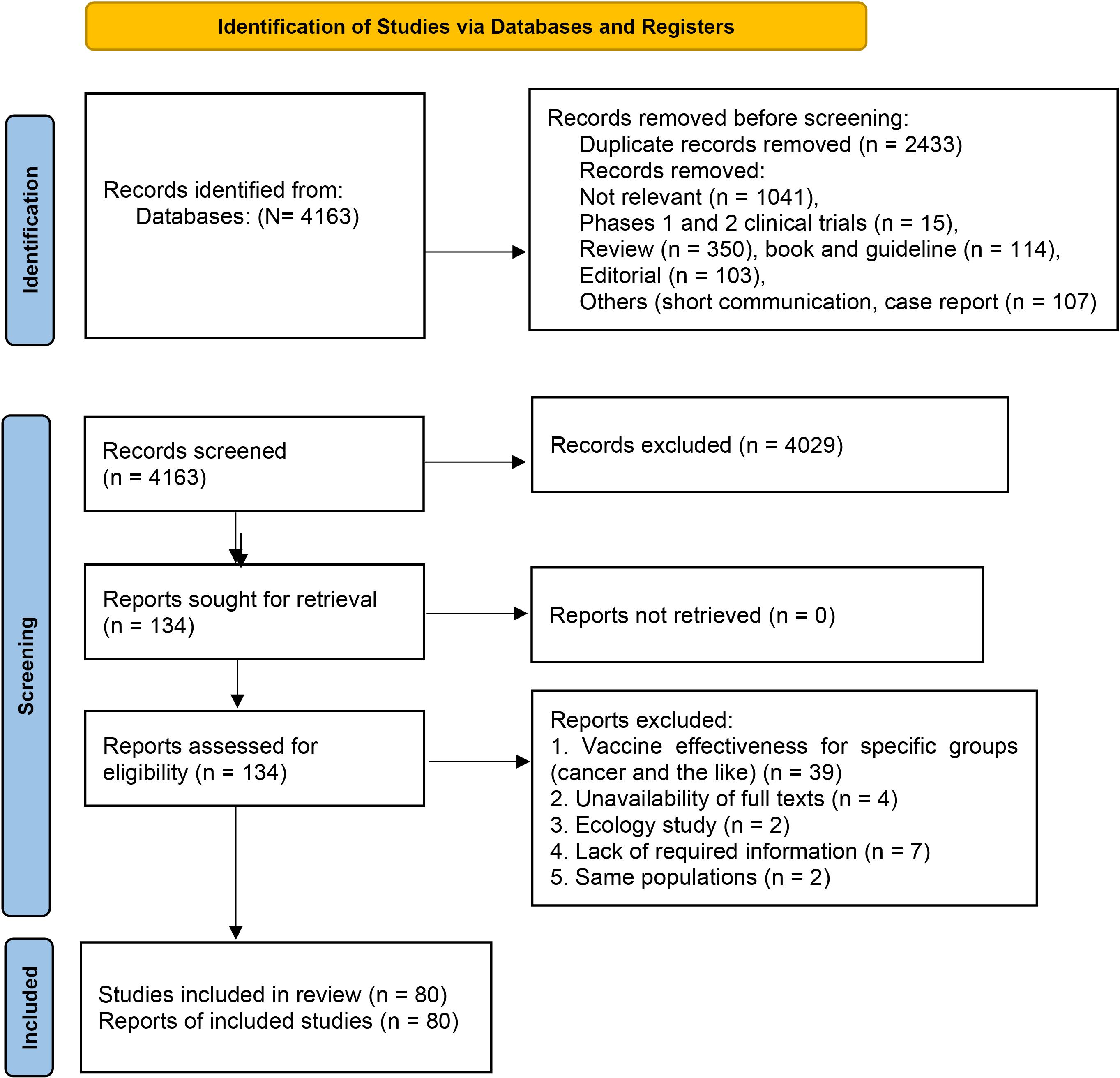
Figure 1.
PRISMA flow diagram for systematic reviews of vaccine effectiveness/effectiveness against SARS-CoV-2 infection. Note. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2
.
PRISMA flow diagram for systematic reviews of vaccine effectiveness/effectiveness against SARS-CoV-2 infection. Note. PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2
The mean ± standard deviation (SD) follow-up times since the booster dose in studies with the UNVCG were 12.6 (± 0.6) and 8.0 (± = 0.3) weeks for the Omicron and Delta variants, respectively. In studies with the complete vaccinated control group, the mean follow-up times after the booster dose were 13.7 (± 0.7) and 7.2 (± 0.5) weeks for the Omicron and Delta variants, respectively.
Among the 80 studies, 18 reported VE at only one time point. These studies were separately analyzed and excluded from the VE time trend analysis over the follow-up period. In addition, 11 of these 18 studies were UNVCG, with a mean follow-up time of 20.1 weeks (SD = 1.4) against the Omicron variant and 15.6 weeks (SD = 1.9) against the Delta variant. In 11 studies with UNVCG, the pooled VE against the Omicron variant was 73.2% (95% CI: 52.6–84.8) for the symptomatic infection and 86.5% (95% CI: 77.0–92.1) for hospitalization. The pooled VE against the Delta variant was 75.7 (95% CI: 73.3–77.8) and 92.5 (95% CI: 89.0–96.9) for symptomatic infection and hospitalization, respectively.
The other 7 out of 18 studies were C2DCG, with a mean follow-up time of 35.1 weeks (SD = 2.9) and 17.7 weeks (SD = 1.9) against the Omicron and Delta variants, respectively. In the seven studies with C2DCG, the pooled VE against the Omicron variant was 68.5 (95% CI: 58.9–75.8) for the symptomatic infection and 85.2 (95% CI: 72.7–92.0) for hospitalization. Moreover, the pooled VE against the Delta variant was 91.9 (95% CI: 88.5–94.3) and 86.7 (95% CI: 64.5–95.0) for symptomatic infection and hospitalization, respectively. There were not enough studies with death as the outcome to evaluate VE.
Overall pooled vaccine effectiveness
VE in studies with the UNVCG: The overall VE (across all time points) for symptomatic infection was 54.0% (95% CI: 51.0–57.0) and 91.0% (95% CI: 90.0–92.0) against the Omicron and Delta variants, respectively (Supplementary file 1, Figures S1 and S2). The VE for hospitalization was 74.0% (95% CI: 65.0–80.0) and 92.0% (95% CI: 90.0–94.0) (Supplementary file 1, Figures S3 and S4). Additionally, the VE for death was 80.0 (95% CI: 57.0–91.0) and 85.0 (95% CI: 78.0–90.0) against the Omicron and Delta variants, respectively (Supplementary file 1, Figures S5 and S6).
VE in studies with the UNVCG:The VE for symptomatic infection was 44.0% (95% CI: 40.0–48.0) and 86.0% (95% CI: 82.0–89.0), and it was 68.0% (95% CI: 63.0–72.0) and 90.0% (95% CI: 82.0–94.0) for hospitalization against the Omicron and Delta variants, respectively. There were not enough studies with death as the outcome for VE evaluation.
Vaccine effectiveness trend in studies with the unvaccinated control group
Symptomatic infection: Multivariate and univariate meta-analyses showed a tendency toward a decrease in long-term VE for symptomatic infection. The mean VE against Delta reached a peak of 91.8% (95% CI: 87.5–94.6) after eight weeks and started to decline with a VE equal to 76.5% (95% CI: 39.0–90.9) after 24 weeks after the booster dose. Furthermore, the effectiveness of the booster dose against the Omicron variant demonstrated a non-linear trend with a peak of 67.3% (95% CI: 58.1–74.4) after eight weeks and decreased to 27.1% (95 CI: 23.9–30.1) after 25 weeks after the booster dose (Table 1).
Table 1.
Meta-analysis results for COVID-19 vaccine effectiveness*
|
Time since booster dose
|
Time 1
(1 week)
|
Time 4
(4 weeks)
|
Time 8
(8 weeks)
|
Time 12
(12 weeks)
|
Time 16
(16 weeks)
|
Time 20
(20 weeks)
|
Time 24
(24 weeks)
|
Time 25
(25≥weeks)
|
| Symptomatic infection in the Delta predominant period (95% CI) |
| Univariate analysis |
78.0 (67.0, 85.0) |
92.0 (90.0, 94.0) |
95.0 (94.0, 96.0) |
93.0 (92.0, 94.0) |
90.0 (89.0, 91.0) |
85.0 (73.0, 91.0) |
79.0 (47.0, 92.0) |
|
| Multivariate analysis |
66.0 (45.4, 78.8) |
90.0 (86.6, 92.5) |
91.8 (87.5, 94.6) |
90.1 (86.1,92.9) |
89.6 (89.1, 90.1) |
80.1 (77.3, 82.5) |
76.5 (39.0, 90.9) |
|
| Number of studies |
18 |
26 |
20 |
28 |
18 |
5 |
2 |
|
| Hospitalization |
| Univariate analysis |
92.0 (88.0, 94.0) |
93.0 (88, 96) |
94.0 (88.0, 97.0) |
93 (87.0, 96.0) |
89.0 (76.0, 95.0) |
87.0 (78.0, 92.0) |
|
|
| Multivariate analysis |
82.0 (72.4, 88.2) |
94.8 (92.2, 96.5) |
96.0 (93.3, 97.6) |
96.0 (93.5, 97.5) |
96.9 (94.1, 98.4) |
93.3 (89.2, 95.9) |
|
|
| Number of studies |
11 |
14 |
16 |
17 |
11 |
5 |
|
|
| Death |
| Univariate analysis |
90.0 (83.0, 94.0) |
90.0 (80.0, 95.0) |
81.0 (49.0, 93.0) |
83.0 (51.0, 94.0) |
62.0 (-40, 86.0) |
71.0 (30.0, 88.0) |
|
|
| Multivariate analysis |
79.9 (61.5, 89.5) |
94.1 (89.4, 96.8) |
95.1 (89.0, 97.8) |
96.2 (88.8, 98.7) |
88.0 (67.8, 95.6) |
90.6 (74.1, 96.6) |
|
|
| Number of studies |
6 |
10 |
12 |
13 |
7 |
4 |
|
|
| Symptomatic infection in the Omicron predominant period (95% CI) |
| Univariate analysis |
53.0 (41.0, 63.0) |
61.0 (57.0, 65.0) |
58.0 (49.0, 65.0) |
56.0 (51.0, 62.0) |
46.0 (33.0, 57.0) |
56.0 (52.0, 59.0) |
46.0 (43.0, 49.0) |
29.0 (22.0, 35.0) |
| Multivariate analysis |
45.4 (22.7, 61.4) |
62.1 (56.7, 66.8) |
67.3 (58.1, 74.4) |
59.0 (49.8, 66.4) |
55.4 (41.9, 65.8) |
42.7 (27.4, 54.8) |
46.1 (43.5, 48.5) |
27.1 (23.9, 30.1) |
| Number of studies |
12 |
19 |
18 |
28 |
10 |
4 |
2 |
11 |
| Hospitalization |
| Univariate analysis |
85.0 (3.0, 98.0) |
89.0 (83.0, 93.0) |
86.0 (77.0, 91.0) |
81.0 (54.0, 92.0) |
53.0 (-54.0, 86.0) |
77.0 (-4.0, 95.0) |
52.0 (-13.0, 80.0) |
45.0 (22.0, 61.0) |
| Multivariate analysis |
60.9 (5.7, 83.8) |
90.8 (85.7, 94.0) |
87.9 (79.0, 93.0) |
81.1 (67.0, 89.2) |
88.2 (75.4, 94.4) |
81.9 (66.6, 90.2) |
80.7 (63.1, 89.9) |
73.4 (51.6, 85.4) |
| Number of studies |
2 |
6 |
12 |
8 |
3 |
5 |
4 |
16 |
Note. CI: Confidence interval; COVID: Coronavirus disease. *The VE of COVID booster dose vaccine compared to the unvaccinated group by endpoints (Outcomes).
Hospitalization: The mean of VE over time in the delta period looks more stable than in the omicron variant period. The mean of VE reached a peak of 96.0% (95% CI: 93.3–97.6) after eight weeks and started to decrease with a VE equal to 93.3% (95% CI: 89.2–95.9) after 20 weeks after the booster dose against Delta. In the Omicron period, the univariate and multivariate models had different estimates. Multivariate models showed a VE of 90.8% (95% CI: 85.7–94.0) against the Omicron variant at four weeks and decreased to 73.4% (95% CI: 51.6–85.4) after 25 weeks after the booster dose (Table 1).
Death: For death, multivariate results revealed a more stable trend, which was 95.1% (95% CI: 89.0–97.8) after eight weeks and then reached a VE equal to 90.6% (95% CI: 74.1–96.6) after 20 weeks after the booster dose (Table 1). There were insufficient studies with death as a result to estimate VE time trends relative to Omicron.
Vaccine effectiveness trend in studies with complete two doses of the vaccine control group
Symptomatic infection: The results indicated a tendency for a decrease in the long-term effectiveness of the vaccine for the studied outcomes (Table 2). For example, a multivariate meta-analysis showed a decreasing trend of 58.7% (95% CI: 53.5–63.3) for the VE against the Omicron variant after four weeks, which declined to 19.1% (95% CI: 6.0–30.3) after 25 weeks after the booster dose.
Table 2.
Meta-analysis results for vaccine effectiveness*
|
Time Since the Booster Dose
|
Time 1 (1 week)
|
Time 4 (4 weeks)
|
Time 8 (8 weeks)
|
Time 12 (12 weeks)
|
Time 16 (16 weeks)
|
Time 20 (20 weeks)
|
Time 24 (24 weeks)
|
Time 25 (≥25 weeks)
|
| Symptomatic infection in the Delta predominant period (95% CI) |
| Univariate analysis |
80.0 (60.0, 90.0) |
84.0 (67.0, 92.0) |
91.0 (85.0, 95.0) |
85.0 (80.0, 88.0) |
88.0 (81.0, 92.0) |
|
|
|
| Multivariate analysis |
36.1 (-26.6, 67.7) |
77.1 (59.5, 87.0) |
91.4 (85.3, 95.0) |
81.9 (74.5, 87.1) |
70.5 (51.1, 82.2) |
|
|
|
| Number of studies |
12 |
15 |
4 |
22 |
5 |
|
|
|
| Hospitalization |
| Univariate analysis |
87.0 (81.0, 91.0) |
90.0 (78.0, 96.0) |
89.0 (80.0, 94.0) |
89.0 (-28.0, 99.0) |
|
|
|
|
| Multivariate analysis |
81.9 (69.2, 89.3) |
81.6 (52.6, 92.8) |
89.3 (80.2, 94.3) |
84.1 (8.8, 97.2) |
|
|
|
|
| Number of studies |
1 |
4 |
2 |
2 |
|
|
|
|
| Symptomatic infection in the Omicron predominant period (95% CI) |
| Univariate analysis |
63.0 (55.0-70.0) |
58.0 (53.0, 63.0) |
45.0 (41.0, 50.0) |
50.0 (43.0, 56.0) |
37 (30.0, 44.0) |
21.0 (16.0, 25.0) |
31.0 (21.0, 40.0) |
5.0 (-7.0, 16.0) |
| Multivariate analysis |
55.2 (44.7, 63.8) |
58.7 (53.5, 63.3) |
51.2 (46.4, 55.6) |
47.1 (39.5, 53.8) |
37.2 (29.9, 43.7) |
26.2 (21.7, 30.5) |
31.5 (21.9, 40.0) |
19.1 (6.0, 30.3) |
| Number of studies |
8 |
20 |
30 |
36 |
9 |
7 |
8 |
19 |
| Hospitalization |
| Univariate analysis |
39.0 (2.0, 62.0) |
76.0 (65.0, 83.0) |
76.0 (69.0, 82.0) |
61.0 (54.0, 68.0) |
53.0 (36.0, 66.0) |
82.0 (69.0, 90.0) |
67.0 (52.0, 77.0) |
62.0 (47.0, 73.0) |
| Multivariate analysis |
31.6 (-12.2, 58.3) |
73.0 (61.6, 81.1) |
72.6 (65.2,78.4) |
66.7 (71.5, 61.0) |
60.7 (54.2, 66.3) |
67.9 (58.2, 75.3) |
66.6 (45.3, 79.7) |
49.1 (25.4, 65.3) |
| Number of studies |
3 |
5 |
12 |
11 |
5 |
6 |
3 |
12 |
Note. COVID: Coronavirus disease; CI: Confidence interval; * The COVID booster dose vaccine compared to the complete vaccinated group.
Hospitalization: Based on the results, there was a decrease in VE over time for hospitalization (Table 2); for example, it reached a peak of 73.0% (95% CI: 61.6–81.1) against Omicron at week eight and decreased to 49.1% (95% CI: 35.4–65.3) after 25 weeks after the booster dose in multivariate analyses. A few studies reported a VE of 84.1% (95% CI: 8.8–97.2) against the Delta variant for the first 12 weeks.
Death: The outcome of death in the complete vaccinated control group was not analyzed due to the small number of studies.
Publication bias
Publication bias was not found with Begg’s test, but Egger’s tests demonstrated publication bias in the analysis of VE in some subgroups; no significant changes were observed after using the trim-and-fill method.
Discussion
This review focused on investigating the effectiveness of the booster dose (third dose) of COVID-19 vaccines in terms of the two control groups for the outcomes of death, hospitalization, and symptomatic infection separately in periods that each variant of Omicron or Delta was predominant.
Based on the results of our study, the overall VE of the booster dose against the Omicron variant for the outcomes of symptomatic infection and hospitalization was 54% and 74% in the UNVCG, as well as 44% and 68% in the completed vaccinated control group, respectively. It was found that the VE of the booster dose for the outcomes of symptomatic infection and hospitalization was higher in studies with the UNVCG compared to the complete vaccinated control group. It was expected that the additive effect of the booster dose on previous vaccination would to be more obvious when the comparison group was unvaccinated, and the obtained data confirmed this issue.
The overall VE of the booster dose against Delta for the outcomes of death, hospitalization, and symptomatic infection in the non-vaccinated control group was 85.0%, 92.0%, and 91.0%, respectively, and in the complete vaccinated control group, it was 86.0% and 90.0% for hospitalization and symptomatic infection. In studies with the complete vaccinated control group, previous vaccination provided some prevention against the outcomes, and the effectiveness of the vaccine in the booster arm compared to this control group was lower in comparison with the studies with the UNVCG.
This review revealed that the VE reached its peak 4 and 8 weeks after the booster dose for the studied outcome against Omicron and Delta, respectively, and then the VE started to decrease. Our findings demonstrated a small but significant decrease in VE over the follow-up periods against the Delta variant, but this downward trend was significantly stronger against the Omicron variant, especially for symptomatic infection and hospitalization in both control groups. These results are in line with those of the study by Patalon et al,45 showing that in the Omicron variant, the VE against infection decreased from 53.4% four weeks after vaccination to 16.5% twelve weeks after vaccination. This downward trend can be justified considering the nature of this type of variant, whose its mutagenic and contagious abilities can change over time. Rana et al94 reported that the Omicron variant has a significant amount of mutation and can have a higher transmission rate than the other variants, which can impair the effectiveness of diagnostic equipment for previous variants and the effectiveness of vaccination. Yu et al95 estimated the time-varying transmissibility and the relative transmissibility of Beta, Delta, and Omicron variants. They found that the transmissibility of the Omicron variant was clearly greater than that of the other two variants.
According to the criteria recommended by the World Health Organization, the adequate effectiveness response of the COVID-19 vaccine indicated at least 70.0% against symptomatic infection and at least 90.0% against hospitalization and death.96,97 Our results are consistent with those of the study performed by Wu et al,97 indicating the lack of effectiveness of the vaccine against the Omicron variants in the UNVCG for symptomatic infection, but the reported effectiveness in our review reached the recommended value of 90.0% at the peak of VE after 4 weeks and then fell below the recommended value in all follow-up periods in the long term. In the completed control group, the recommended criteria were not met for the studied outcomes against Omicron. The recommended effectiveness for all outcomes in the two control groups was observed in the Delta variant.
The present study has some limitations that should be mentioned. Firstly, although the researchers attempted to control the amount of heterogeneity in this study with different methods, there was still a significant amount of heterogeneity in our study, which is probably due to the existence of variables that were influential in heterogeneity between studies and were not considered in our study. Secondly, the included studies in this meta-analysis review were different in terms of the study setting, different periods, locations, and populations, and the real overall effect could have been affected by this heterogeneity. Thirdly, some variables, such as previous COVID-19 infection and age, were not well-defined in the studies or were missing, and the authors did not cooperate in sharing information; thus, we could not perform adjusted multivariate analysis to estimate VE by controlling variables. Fourth, many outcomes, especially symptomatic infection, have been reported in some studies based on self-reported forms, which could have affected the overall effect on the results. Finally, we could not assess the role of the different sub-variants of Omicron due to a lack of sufficient studies or reports on the sub-variants of Omicron in different studies.
Highlights
-
The mean of VE for hospitalization over time started to decrease after four and eight weeks against Omicron and Delta, respectively.
-
The VE reached a peak after eight weeks and began to decline with a VE after 20 weeks after the booster dose against Delta.
-
The VE reached a peak after four weeks and started to decline with a VE after 25 weeks after the booster dose against Omicron.
-
The early protection levels were lower in Omicron variants, and the VE decrease over time was stronger in the Omicron variant in comparison to the Delta variant.
Conclusion
Our study confirmed a tendency to decrease effectiveness over time based on outcomes and variants. The early protection levels were lower in Omicron variants, and the VE decrease over time was stronger in the Omicron variant in comparison to Delta.
Authors’ Contribution
Conceptualization: Farideh Mostafavi, Mansour Bahahrdoust, and Seyed Saeed Hashemi Nazari.
Data curation: Farideh Mostafavi, Mansour Bahahrdoust, and Alireza Amirabadizadeh.
Formal analysis: Farideh Mostafavi, Fransisco Sera, Manochehr Karami, and Seyed Saeed Hashemi Nazari.
Investigation: Manochehr Karami and Seyed Saeed Hashemi Nazari.
Methodology: Farideh Mostafavi, Francesco Sera, and Seyed Saeed Hashemi Nazari.
Project administration: Seyed Saeed Hashemi Nazari.
Software: Farideh Mostafavi, Francesco Sera, Paddy Ssentongod, and Seyed Saeed Hashemi Nazari.
Supervision: Seyed Saeed Hashemi Nazari.
Validation: Manochehr Karami and Seyed Saeed Hashemi Nazari.
Visualization: Manochehr Karami and Seyed Saeed Hashemi Nazari.
Writing–original draft: Farideh Mostafavi, Mansour Bahahrdoust, and Alireza Amirabadizadeh.
Writing–review & editing: Manochehr Karami, Sepehr Allahyari, and Seyed Saeed Hashemi Nazari.
Competing Interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Funding
Not applicable.
Supplementary Files
Supplementary file 1 contains Figures S1-S6.
(pdf)
References
- Dinleyici EC, Borrow R, Safadi MAP, van Damme P, Munoz FM. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccin Immunother 2021; 17(2):400-7. doi: 10.1080/21645515.2020.1804776 [Crossref] [ Google Scholar]
- Rudolph A, Mitchell J, Barrett J, Sköld H, Taavola H, Erlanson N. Global safety monitoring of COVID-19 vaccines: how pharmacovigilance rose to the challenge. Ther Adv Drug Saf 2022; 13:20420986221118972. doi: 10.1177/20420986221118972 [Crossref] [ Google Scholar]
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022; 28(4):831-7. doi: 10.1038/s41591-022-01699-1 [Crossref] [ Google Scholar]
- Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med 2021; 27(12):2127-35. doi: 10.1038/s41591-021-01548-7 [Crossref] [ Google Scholar]
- Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis 2021; 21(11):1539-48. doi: 10.1016/s1473-3099(21)00330-3 [Crossref] [ Google Scholar]
- Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv [Preprint]. October 6, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.09.15.21263583v2.
- Morgan RL, Florez ID. Principles of systematic reviews and meta-analyses. Methods Mol Biol 2022; 2345:1-15. doi: 10.1007/978-1-0716-1566-9_1 [Crossref] [ Google Scholar]
- Penson D, Krishnaswami S, Jules A, Seroogy J, McPheeters M. Newcastle-Ottawa Quality Assessment form for Cohort Studies. Ottawa: Ottawa Hospital Research Institute; 2012.
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. p. 205-28.
- Ishak KJ, Platt RW, Joseph L, Hanley JA, Caro JJ. Meta-analysis of longitudinal studies. Clin Trials 2007; 4(5):525-39. doi: 10.1177/1740774507083567 [Crossref] [ Google Scholar]
- Sera F, Armstrong B, Blangiardo M, Gasparrini A. An extended mixed-effects framework for meta-analysis. Stat Med 2019; 38(29):5429-44. doi: 10.1002/sim.8362 [Crossref] [ Google Scholar]
- Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore) 2019; 98(23):e15987. doi: 10.1097/md.0000000000015987 [Crossref] [ Google Scholar]
- Gjerdevik M, Heuch I. Improving the error rates of the Begg and Mazumdar test for publication bias in fixed effects meta-analysis. BMC Med Res Methodol 2014; 14:109. doi: 10.1186/1471-2288-14-109 [Crossref] [ Google Scholar]
- Moreira ED Jr, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A. Safety and efficacy of a third dose of BNT162b2 COVID-19 vaccine. N Engl J Med 2022; 386(20):1910-21. doi: 10.1056/NEJMoa2200674 [Crossref] [ Google Scholar]
- Wang XY, Mahmood SF, Jin F, Cheah WK, Ahmad M, Sohail MA. Efficacy of heterologous boosting against SARS-CoV-2 using a recombinant interferon-armed fusion protein vaccine (V-01): a randomized, double-blind and placebo-controlled phase III trial. Emerg Microbes Infect 2022; 11(1):1910-9. doi: 10.1080/22221751.2022.2088406 [Crossref] [ Google Scholar]
- Cerqueira-Silva T, Katikireddi SV, de Araujo Oliveira V, Flores-Ortiz R, Júnior JB, Paixão ES. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med 2022; 28(4):838-43. doi: 10.1038/s41591-022-01701-w [Crossref] [ Google Scholar]
- Cerqueira-Silva T, de Araujo Oliveira V, Paixão ES, Júnior JB, Penna GO, Werneck GL. Duration of protection of CoronaVac plus heterologous BNT162b2 booster in the Omicron period in Brazil. Nat Commun 2022; 13(1):4154. doi: 10.1038/s41467-022-31839-7 [Crossref] [ Google Scholar]
- Huang Z, Xu S, Liu J, Wu L, Qiu J, Wang N. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA 2 variant infection, severe illness, and death. BMC Med 2022; 20(1):400. doi: 10.1186/s12916-022-02606-8 [Crossref] [ Google Scholar]
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021; 398(10316):2093-100. doi: 10.1016/s0140-6736(21)02249-2 [Crossref] [ Google Scholar]
- Tenforde MW, Patel MM, Gaglani M, Ginde AA, Douin DJ, Talbot HK. Effectiveness of a third dose of Pfizer-BioNTech and Moderna vaccines in preventing COVID-19 hospitalization among immunocompetent and immunocompromised adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 2022; 71(4):118-24. doi: 10.15585/mmwr.mm7104a2 [Crossref] [ Google Scholar]
- Korves C, Izurieta HS, Smith J, Zwain GM, Powell EI, Balajee A. Relative effectiveness of booster vs 2-dose mRNA COVID-19 vaccination in the Veterans Health Administration: self-controlled risk interval analysis. Vaccine 2022; 40(33):4742-7. doi: 10.1016/j.vaccine.2022.06.047 [Crossref] [ Google Scholar]
- Silva-Valencia J, Soto-Becerra P, Escobar-Agreda S, Fernandez-Navarro M, Elorreaga OA, Mayta-Tristán P. Relative vaccine effectiveness of the booster dose of COVID-19 vaccine for preventing death in individuals with a primary regimen based on the BBIBP-CorV, ChAdOx1-S, or BNT162b2 vaccines during the Omicron wave in Peru: a nested case-control study using national population data. Vaccine 2022; 40(45):6512-9. doi: 10.1016/j.vaccine.2022.09.066 [Crossref] [ Google Scholar]
- Martínez-Baz I, Trobajo-Sanmartín C, Miqueleiz A, Casado I, Navascués A, Burgui C. Risk reduction of hospitalisation and severe disease in vaccinated COVID-19 cases during the SARS-CoV-2 variant Omicron BA1-predominant period, Navarre, Spain, January to March 2022. Euro Surveill 2023; 28(5):2200337. doi: 10.2807/1560-7917.es.2023.28.5.2200337 [Crossref] [ Google Scholar]
- Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA1 and BA2 subvariants in Qatar. Nat Commun 2022; 13(1):3082. doi: 10.1038/s41467-022-30895-3 [Crossref] [ Google Scholar]
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E. COVID-19 vaccine effectiveness against the Omicron (B11529) variant. N Engl J Med 2022; 386(16):1532-46. doi: 10.1056/NEJMoa2119451 [Crossref] [ Google Scholar]
- Fabiani M, Puopolo M, Filia A, Sacco C, Mateo-Urdiales A, Spila Alegiani S. Effectiveness of an mRNA vaccine booster dose against SARS-CoV-2 infection and severe COVID-19 in persons aged ≥ 60 years and other high-risk groups during predominant circulation of the Delta variant in Italy, 19 July to 12 December 2021. Expert Rev Vaccines 2022; 21(7):975-82. doi: 10.1080/14760584.2022.2064280 [Crossref] [ Google Scholar]
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA 2022; 327(7):639-51. doi: 10.1001/jama.2022.0470 [Crossref] [ Google Scholar]
- Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71(4):139-45. doi: 10.15585/mmwr.mm7104e3 [Crossref] [ Google Scholar]
- Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 states, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71(7):255-63. doi: 10.15585/mmwr.mm7107e2 [Crossref] [ Google Scholar]
- Natarajan K, Prasad N, Dascomb K, Irving SA, Yang DH, Gaglani M. Effectiveness of homologous and heterologous COVID-19 booster doses following 1 Ad26COV2S (Janssen [Johnson & Johnson]) vaccine dose against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults - VISION Network, 10 states, December 2021-March 2022. MMWR Morb Mortal Wkly Rep 2022; 71(13):495-502. doi: 10.15585/mmwr.mm7113e2 [Crossref] [ Google Scholar]
- Young-Xu Y, Zwain GM, Izurieta HS, Korves C, Powell EI, Smith J. Effectiveness of mRNA COVID-19 vaccines against Omicron and Delta variants in a matched test-negative case-control study among US veterans. BMJ Open 2022; 12(8):e063935. doi: 10.1136/bmjopen-2022-063935 [Crossref] [ Google Scholar]
- Suphanchaimat R, Nittayasoot N, Jiraphongsa C, Thammawijaya P, Bumrungwong P, Tulyathan A. Real-world effectiveness of mix-and-match vaccine regimens against SARS-CoV-2 Delta variant in Thailand: a nationwide test-negative matched case-control study. Vaccines (Basel) 2022; 10(7):1080. doi: 10.3390/vaccines10071080 [Crossref] [ Google Scholar]
- Link-Gelles R, Levy ME, Gaglani M, Irving SA, Stockwell M, Dascomb K. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 Omicron BA1 and BA2/BA2121 sublineages predominated - VISION Network, 10 states, December 2021-June 2022. MMWR Morb Mortal Wkly Rep 2022; 71(29):931-9. doi: 10.15585/mmwr.mm7129e1 [Crossref] [ Google Scholar]
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022; 28(4):831-7. doi: 10.1038/s41591-022-01699-1 [Crossref] [ Google Scholar]
- Kirsebom FC, Andrews N, Sachdeva R, Stowe J, Ramsay M, Lopez Bernal J. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. Nat Commun 2022; 13(1):7688. doi: 10.1038/s41467-022-35168-7 [Crossref] [ Google Scholar]
- Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA. Waning of vaccine effectiveness against moderate and severe COVID-19 among adults in the US from the VISION Network: test negative, case-control study. BMJ 2022; 379:e072141. doi: 10.1136/bmj-2022-072141 [Crossref] [ Google Scholar]
- Tan CY, Chiew CJ, Pang D, Lee VJ, Ong B, Lye DC. Vaccine effectiveness against Delta, Omicron BA1, and BA2 in a highly vaccinated Asian setting: a test-negative design study. Clin Microbiol Infect 2023; 29(1):101-6. doi: 10.1016/j.cmi.2022.08.002 [Crossref] [ Google Scholar]
- Cerqueira-Silva T, Shah SA, Robertson C, Sanchez M, Katikireddi SV, de Araujo Oliveira V. Effectiveness of mRNA boosters after homologous primary series with BNT162b2 or ChAdOx1 against symptomatic infection and severe COVID-19 in Brazil and Scotland: a test-negative design case-control study. PLoS Med 2023; 20(1):e1004156. doi: 10.1371/journal.pmed.1004156 [Crossref] [ Google Scholar]
- Link-Gelles R, Levy ME, Natarajan K, Reese SE, Naleway AL, Grannis SJ. Estimation of COVID-19 mRNA vaccine effectiveness and COVID-19 illness and severity by vaccination status during Omicron BA4 and BA5 sublineage periods. JAMA Netw Open 2023; 6(3):e232598. doi: 10.1001/jamanetworkopen.2023.2598 [Crossref] [ Google Scholar]
- Intawong K, Chariyalertsak S, Chalom K, Wonghirundecha T, Kowatcharakul W, Thongprachum A. Effectiveness of heterologous third and fourth dose COVID-19 vaccine schedules for SARS-CoV-2 infection during Delta and Omicron predominance in Thailand: a test-negative, case-control study. Lancet Reg Health Southeast Asia 2023; 10:100121. doi: 10.1016/j.lansea.2022.100121 [Crossref] [ Google Scholar]
- Tamandjou Tchuem CR, Auvigne V, Vaux S, Montagnat C, Paireau J, Monnier Besnard S. Vaccine effectiveness and duration of protection of COVID-19 mRNA vaccines against Delta and Omicron BA1 symptomatic and severe COVID-19 outcomes in adults aged 50 years and over in France. Vaccine 2023; 41(13):2280-8. doi: 10.1016/j.vaccine.2023.02.062 [Crossref] [ Google Scholar]
- Grewal R, Nguyen L, Buchan SA, Wilson SE, Nasreen S, Austin PC. Effectiveness of mRNA COVID-19 vaccine booster doses against Omicron severe outcomes. Nat Commun 2023; 14(1):1273. doi: 10.1038/s41467-023-36566-1 [Crossref] [ Google Scholar]
- Arashiro T, Arima Y, Muraoka H, Sato A, Oba K, Uehara Y. Coronavirus disease 19 (COVID-19) vaccine effectiveness against symptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during Delta-dominant and Omicron-dominant periods in Japan: a multicenter prospective case-control study (factors associated with SARS-CoV-2 infection and the effectiveness of COVID-19 vaccines study). Clin Infect Dis 2023; 76(3):e108-15. doi: 10.1093/cid/ciac635 [Crossref] [ Google Scholar]
- Patalon T, Gazit S, Pitzer VE, Prunas O, Warren JL, Weinberger DM. Odds of testing positive for SARS-CoV-2 following receipt of 3 vs 2 doses of the BNT162b2 mRNA vaccine. JAMA Intern Med 2022; 182(2):179-84. doi: 10.1001/jamainternmed.2021.7382 [Crossref] [ Google Scholar]
- Patalon T, Saciuk Y, Peretz A, Perez G, Lurie Y, Maor Y. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun 2022; 13(1):3203. doi: 10.1038/s41467-022-30884-6 [Crossref] [ Google Scholar]
- Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. Severity of Omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis 2022; 22(7):959-66. doi: 10.1016/s1473-3099(22)00141-4 [Crossref] [ Google Scholar]
- Plumb ID, Feldstein LR, Barkley E, Posner AB, Bregman HS, Hagen MB. Effectiveness of COVID-19 mRNA vaccination in preventing COVID-19-associated hospitalization among adults with previous SARS-CoV-2 infection - United States, June 2021-February 2022. MMWR Morb Mortal Wkly Rep 2022; 71(15):549-55. doi: 10.15585/mmwr.mm7115e2 [Crossref] [ Google Scholar]
- Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med 2022; 387(1):21-34. doi: 10.1056/NEJMoa2203965 [Crossref] [ Google Scholar]
- Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the Delta variant-dominant pandemic: a test-negative case-control study. Emerg Microbes Infect 2022; 11(1):585-92. doi: 10.1080/22221751.2022.2037398 [Crossref] [ Google Scholar]
- Suah JL, Tng BH, Tok PSK, Husin M, Thevananthan T, Peariasamy KM. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg Microbes Infect 2022; 11(1):1343-5. doi: 10.1080/22221751.2022.2072773 [Crossref] [ Google Scholar]
- Adams K, Rhoads JP, Surie D, Gaglani M, Ginde AA, McNeal T. Vaccine effectiveness of primary series and booster doses against COVID-19 associated hospital admissions in the United States: living test negative design study. BMJ 2022; 379:e072065. doi: 10.1136/bmj-2022-072065 [Crossref] [ Google Scholar]
- Ranzani OT, Hitchings MD, de Melo RL, de França GV, de Fátima Rangel Fernandes C, Lind ML. Effectiveness of an inactivated COVID-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nat Commun 2022; 13(1):5536. doi: 10.1038/s41467-022-33169-0 [Crossref] [ Google Scholar]
- Lind ML, Robertson AJ, Silva J, Warner F, Coppi AC, Price N. Association between primary or booster COVID-19 mRNA vaccination and Omicron lineage BA1 SARS-CoV-2 infection in people with a prior SARS-CoV-2 infection: a test-negative case-control analysis. PLoS Med 2022; 19(12):e1004136. doi: 10.1371/journal.pmed.1004136 [Crossref] [ Google Scholar]
- Surie D, Bonnell L, Adams K, Gaglani M, Ginde AA, Douin DJ. Effectiveness of monovalent mRNA vaccines against COVID-19-associated hospitalization among immunocompetent adults during BA1/BA2 and BA4/BA5 predominant periods of SARS-CoV-2 Omicron variant in the United States - IVY Network, 18 states, December 26, 2021-August 31, 2022. MMWR Morb Mortal Wkly Rep 2022; 71(42):1327-34. doi: 10.15585/mmwr.mm7142a3 [Crossref] [ Google Scholar]
- Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A. Real-life effectiveness of COVID-19 vaccine during the Omicron variant-dominant pandemic: how many booster doses do we need?. Emerg Microbes Infect 2023; 12(1):2174779. doi: 10.1080/22221751.2023.2174779 [Crossref] [ Google Scholar]
- Richterman A, Behrman A, Brennan PJ, O’Donnell JA, Snider CK, Chaiyachati KH. Durability of severe acute respiratory syndrome coronavirus 2 messenger RNA booster vaccine protection against Omicron among healthcare workers with a vaccine mandate. Clin Infect Dis 2023; 76(3):e319-26. doi: 10.1093/cid/ciac454 [Crossref] [ Google Scholar]
- Glatman-Freedman A, Bromberg M, Hershkovitz Y, Sefty H, Kaufman Z, Dichtiar R. Effectiveness of BNT162b2 vaccine booster against SARS-CoV-2 infection and breakthrough complications, Israel. Emerg Infect Dis 2022; 28(5):948-56. doi: 10.3201/eid2805.220141 [Crossref] [ Google Scholar]
- Waxman JG, Makov-Assif M, Reis BY, Netzer D, Balicer RD, Dagan N. Comparing COVID-19-related hospitalization rates among individuals with infection-induced and vaccine-induced immunity in Israel. Nat Commun 2022; 13(1):2202. doi: 10.1038/s41467-022-29858-5 [Crossref] [ Google Scholar]
- Björk J, Bonander C, Moghaddassi M, Rasmussen M, Malmqvist U, Inghammar M, et al. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 Omicron BA.1 and BA.2 subvariants - surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill 2022;27(18). 10.2807/1560-7917.es.2022.27.18.2200322.
- Tartof SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am 2022; 9:100198. doi: 10.1016/j.lana.2022.100198 [Crossref] [ Google Scholar]
- Starrfelt J, Danielsen AS, Buanes EA, Juvet LK, Lyngstad TM, Rø G. Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July-November 2021. BMC Med 2022; 20(1):278. doi: 10.1186/s12916-022-02480-4 [Crossref] [ Google Scholar]
- Miyauchi S, Hiyama T, Nakano Y, Yoshida M, Yoshino A, Miyake Y. Real-world effectiveness of a booster dose of the COVID-19 vaccines among Japanese university students. Vaccines (Basel) 2022; 10(8):1283. doi: 10.3390/vaccines10081283 [Crossref] [ Google Scholar]
- Akaishi T, Kushimoto S, Katori Y, Sugawara N, Egusa H, Igarashi K. Effectiveness of third vaccine dose for coronavirus disease 2019 during the Omicron variant pandemic: a prospective observational study in Japan. Sci Rep 2022; 12(1):13589. doi: 10.1038/s41598-022-17990-7 [Crossref] [ Google Scholar]
- Sonmezer MC, Dizman GT, Erul E, Sahin TK, Saricaoglu T, Alp A. Relative vaccine effectiveness of the third dose of CoronaVac or BNT162b2 following a two-dose CoronaVac regimen: a prospective observational cohort study from an adult vaccine center in Turkey. Vaccines (Basel) 2022; 10(7):1140. doi: 10.3390/vaccines10071140 [Crossref] [ Google Scholar]
- Rothberg MB, Kim P, Shrestha NK, Kojima L, Tereshchenko LG. Protection against the Omicron variant offered by previous severe acute respiratory syndrome coronavirus 2 infection: a retrospective cohort study. Clin Infect Dis 2023; 76(3):e142-7. doi: 10.1093/cid/ciac604 [Crossref] [ Google Scholar]
- Shrotri M, Krutikov M, Nacer-Laidi H, Azmi B, Palmer T, Giddings R. Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Healthy Longev 2022; 3(7):e470-80. doi: 10.1016/s2666-7568(22)00147-7 [Crossref] [ Google Scholar]
- Kiss Z, Wittmann I, Polivka L, Surján G, Surján O, Barcza Z. Nationwide effectiveness of first and second SARS-CoV2 booster vaccines during the Delta and Omicron pandemic waves in Hungary (HUN-VE 2 study). Front Immunol 2022; 13:905585. doi: 10.3389/fimmu.2022.905585 [Crossref] [ Google Scholar]
- Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis 2022; 22(7):1002-10. doi: 10.1016/s1473-3099(22)00146-3 [Crossref] [ Google Scholar]
- Jara A, Undurraga EA, Zubizarreta JR, González C, Pizarro A, Acevedo J. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health 2022; 10(6):e798-806. doi: 10.1016/s2214-109x(22)00112-7 [Crossref] [ Google Scholar]
- Hua Q, Zheng D, Yu B, Tan X, Chen Q, Wang L. Effectiveness of inactivated COVID-19 vaccines against COVID-19 caused by the SARS-CoV-2 Delta and Omicron variants: a retrospective cohort study. Vaccines (Basel) 2022; 10(10):1753. doi: 10.3390/vaccines10101753 [Crossref] [ Google Scholar]
- Kislaya I, Machado A, Magalhães S, Rodrigues AP, Franco R, Leite PP. COVID-19 mRNA vaccine effectiveness (second and first booster dose) against hospitalisation and death during Omicron BA5 circulation: cohort study based on electronic health records, Portugal, May to July 2022. Euro Surveill 2022; 27(37):2200697. doi: 10.2807/1560-7917.es.2022.27.37.2200697 [Crossref] [ Google Scholar]
- Kislaya I, Peralta-Santos A, Borges V, Vieira L, Sousa C, Ferreira B. Comparative complete scheme and booster effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infections with SARS-CoV-2 Omicron (BA1) and Delta (B16172) variants: a case-case study based on electronic health records. Influenza Other Respir Viruses 2023; 17(3):e13121. doi: 10.1111/irv.13121 [Crossref] [ Google Scholar]
- Tsang NN, So HC, Cowling BJ, Leung GM, Ip DK. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 Omicron BA2 in Hong Kong: a prospective cohort study. Lancet Infect Dis 2023; 23(4):421-34. doi: 10.1016/s1473-3099(22)00732-0 [Crossref] [ Google Scholar]
- dos Santos CV, de Noronha TG, Werneck GL, Struchiner CJ, Villela DA. Estimated COVID-19 severe cases and deaths averted in the first year of the vaccination campaign in Brazil: a retrospective observational study. Lancet Reg Health Am 2023; 17:100418. doi: 10.1016/j.lana.2022.100418 [Crossref] [ Google Scholar]
- Marra AR, Miraglia JL, Malheiros DT, Guozhang Y, Teich VD, da Silva Victor E. Effectiveness of heterologous coronavirus disease 2019 (COVID-19) vaccine booster dosing in Brazilian healthcare workers, 2021. Clin Infect Dis 2023; 76(3):e360-6. doi: 10.1093/cid/ciac430 [Crossref] [ Google Scholar]
- Yamal JM, Appana S, Wang M, Leon-Novelo L, Bakota E, Ye Y. Trends and correlates of breakthrough infections with SARS-CoV-2. Front Public Health 2022; 10:856532. doi: 10.3389/fpubh.2022.856532 [Crossref] [ Google Scholar]
- Monge S, Rojas-Benedicto A, Olmedo C, Mazagatos C, José Sierra M, Limia A. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 Omicron (B11529) variant in Spain: a nationwide cohort study. Lancet Infect Dis 2022; 22(9):1313-20. doi: 10.1016/s1473-3099(22)00292-4 [Crossref] [ Google Scholar]
- Oster Y, Benenson S, Nir-Paz R, Buda I, Cohen MJ. The effect of a third BNT162b2 vaccine on breakthrough infections in health care workers: a cohort analysis. Clin Microbiol Infect 2022;28(5):735.e1-735.e3. 10.1016/j.cmi.2022.01.019.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med 2021; 385(15):1393-400. doi: 10.1056/NEJMoa2114255 [Crossref] [ Google Scholar]
- Arbel R, Sergienko R, Hammerman A. BNT162b2 vaccine booster and COVID-19 mortality Reply. N Engl J Med 2022; 386(10):1000-1. doi: 10.1056/NEJMc2120044 [Crossref] [ Google Scholar]
- Ng OT, Marimuthu K, Lim N, Lim ZQ, Thevasagayam NM, Koh V. Analysis of COVID-19 incidence and severity among adults vaccinated with 2-dose mRNA COVID-19 or inactivated SARS-CoV-2 vaccines with and without boosters in Singapore. JAMA Netw Open 2022; 5(8):e2228900. doi: 10.1001/jamanetworkopen.2022.28900 [Crossref] [ Google Scholar]
- Robilotti EV, Whiting K, Lucca A, Poon C, Jani K, McMillen T. Effectiveness of MRNA booster vaccine among healthcare workers in New York City during the Omicron surge, December 2021 to January 2022. Clin Microbiol Infect 2022; 28(12):1624-8. doi: 10.1016/j.cmi.2022.07.017 [Crossref] [ Google Scholar]
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, AlMukdad S, Yassine HM, Al-Khatib HA. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med 2022; 386(19):1804-16. doi: 10.1056/NEJMoa2200797 [Crossref] [ Google Scholar]
- Mehta HB, Li S, Goodwin JS. Effectiveness of COVID-19 booster on the risk of hospitalization among Medicare beneficiaries. Mayo Clin Proc 2022; 97(10):1780-93. doi: 10.1016/j.mayocp.2022.06.029 [Crossref] [ Google Scholar]
- Tang L, Zhang Y, Wang F, Wu D, Qian ZH, Zhang R. Relative vaccine effectiveness against Delta and Omicron COVID-19 after homologous inactivated vaccine boosting: a retrospective cohort study. BMJ Open 2022; 12(11):e063919. doi: 10.1136/bmjopen-2022-063919 [Crossref] [ Google Scholar]
- Ioannou GN, Bohnert ASB, O’Hare AM, Boyko EJ, Maciejewski ML, Smith VA. Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization, and death: a target trial emulation in the Omicron (B11529) variant era. Ann Intern Med 2022; 175(12):1693-706. doi: 10.7326/m22-1856 [Crossref] [ Google Scholar]
- Laake I, Skodvin SN, Blix K, Caspersen IH, Gjessing HK, Juvet LK. Effectiveness of mRNA booster vaccination against mild, moderate, and severe COVID-19 caused by the Omicron variant in a large, population-based, Norwegian cohort. J Infect Dis 2022; 226(11):1924-33. doi: 10.1093/infdis/jiac419 [Crossref] [ Google Scholar]
- Butt AA, Talisa VB, Shaikh OS, Omer SB, Mayr FB. Relative vaccine effectiveness of a severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine booster dose against the Omicron variant. Clin Infect Dis 2022; 75(12):2161-8. doi: 10.1093/cid/ciac328 [Crossref] [ Google Scholar]
- Wong MT, Dhaliwal SS, Balakrishnan V, Nordin F, Norazmi MN, Tye GJ. Effectiveness of booster vaccinations on the control of COVID-19 during the spread of Omicron variant in Malaysia. Int J Environ Res Public Health 2023; 20(2):1647. doi: 10.3390/ijerph20021647 [Crossref] [ Google Scholar]
- Hsu CY, Chang JC, Chen SL, Chang HH, Lin AT, Yen AM. Primary and booster vaccination in reducing severe clinical outcomes associated with Omicron Naïve infection. J Infect Public Health 2023; 16(1):55-63. doi: 10.1016/j.jiph.2022.11.028 [Crossref] [ Google Scholar]
- Kompaniyets L, Wiegand RE, Oyalowo AC, Bull-Otterson L, Egwuogu H, Thompson T. Relative effectiveness of coronavirus disease 2019 vaccination and booster dose combinations among 189 million vaccinated adults during the early severe acute respiratory syndrome coronavirus 2 Omicron period-United States, 1 January 2022 to 31 March 2022. Clin Infect Dis 2023; 76(10):1753-60. doi: 10.1093/cid/ciad063 [Crossref] [ Google Scholar]
- Petrie JG, King JP, McClure DL, Rolfes MA, Meece JK, Pattinson D. Effectiveness of first and second COVID-19 mRNA vaccine monovalent booster doses during a period of circulation of Omicron variant sublineages: December 2021-July 2022. Influenza Other Respir Viruses 2023; 17(3):e13104. doi: 10.1111/irv.13104 [Crossref] [ Google Scholar]
- Chemaitelly H, Ayoub HH, AlMukdad S, Coyle P, Tang P, Yassine HM. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA1 and BA2 subvariants in Qatar. Nat Commun 2022; 13(1):3082. doi: 10.1038/s41467-022-30895-3 [Crossref] [ Google Scholar]
- Rana R, Kant R, Huirem RS, Bohra D, Ganguly NK. Omicron variant: current insights and future directions. Microbiol Res 2022; 265:127204. doi: 10.1016/j.micres.2022.127204 [Crossref] [ Google Scholar]
- Yu Y, Yu Y, Zhao S, He D. A simple model to estimate the transmissibility of the Beta, Delta, and Omicron variants of SARS-COV-2 in South Africa. Math Biosci Eng 2022; 19(10):10361-73. doi: 10.3934/mbe.2022485 [Crossref] [ Google Scholar]
- Caballero N, Nieto MA, Suarez-Zamora DA, Arias-Amaya JS, Duran D, González-Teshima LY, et al. Effectiveness and safety of covid-19 vaccines available in middle-income countries to prevent infection, hospitalisation, and death due to COVID-19: a systematic review and meta-analysis. Preprint. Posted online February 3, 2023. SSRN. 10.2139/ssrn.4344634.
- Wu N, Joyal-Desmarais K, Ribeiro PA, Vieira AM, Stojanovic J, Sanuade C. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med 2023; 11(5):439-52. doi: 10.1016/s2213-2600(23)00015-2 [Crossref] [ Google Scholar]